Welcome to the official website of Sinos (Suzhou) Bioengineering Co., Ltd!
Chlamydia pneumoniae IgG IgM (Colloidal Gold Method)
date:2023-12-19 23:35
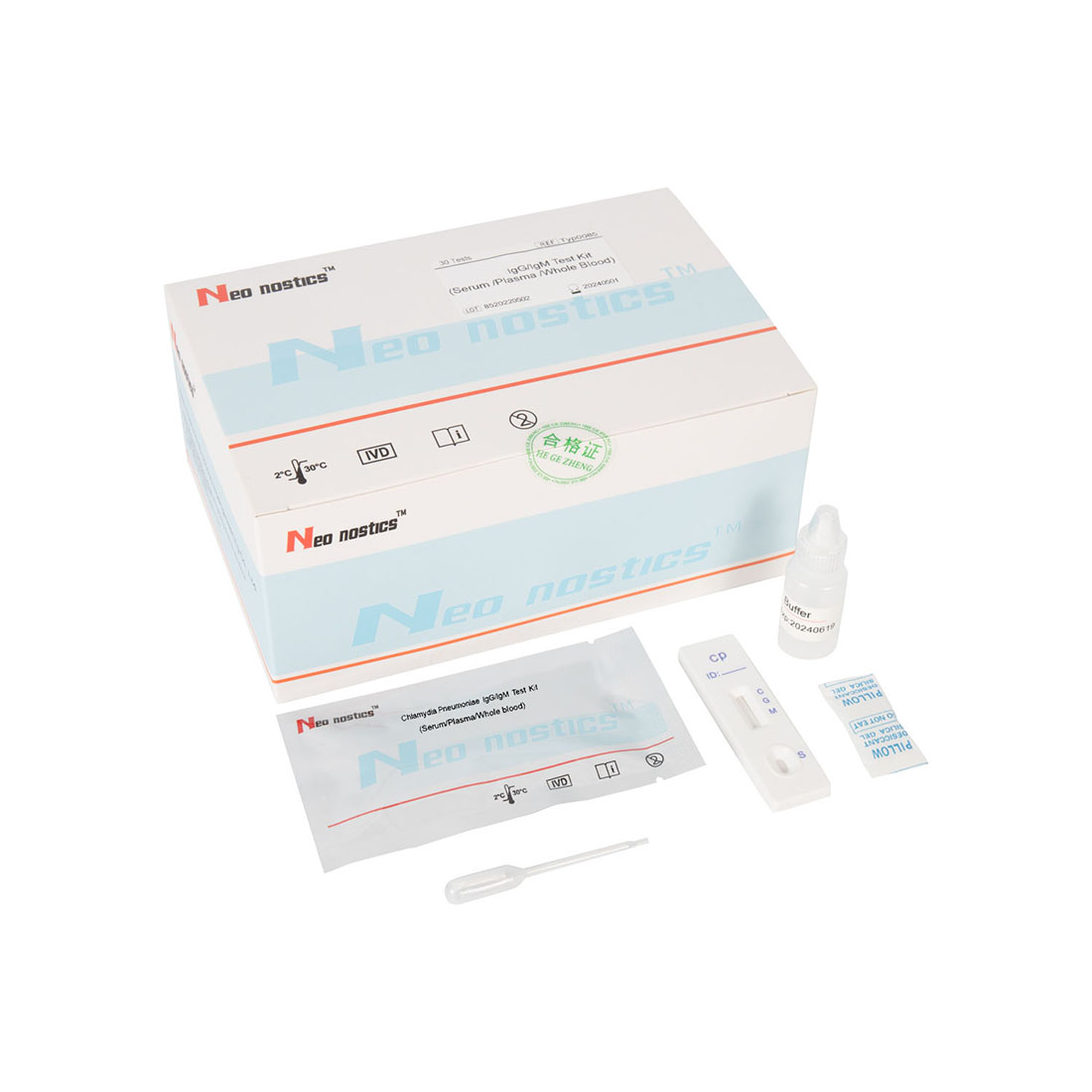
Introduction
This kit is used for in vitro qualitative detection of Chlamydia pneumoniae IgM/IgG antibodies in human venous plasma / serum or whole blood, samples .
Chlamydia pneumoniae(CP) is a microorganism that lies between bacteria and viruses, belonging to the Chlamydia genus of the Chlamydia family. The pathogen is mainly transmitted through the respiratory tract and can cause respiratory infectious diseases, such as bronchitis, pneumonia, etc. In clinical practice, serological tests can generally be used to determine whether one is infected with Chlamydia pneumoniae. If the patient's blood routine test results show normal or low white blood cell count and an increase in lymphocyte proportion, it can be preliminarily diagnosed as Chlamydia pneumoniae infection. IgG antibodies are a type of immunoglobulin produced against Chlamydia pneumoniae. When the body is infected with Chlamydia pneumoniae, specific antibodies are produced in the body, resulting in IgG antibody positivity. Therefore, a positive IgG antibody and a negative IgM antibody for Chlamydia pneumoniae indicate that the patient has previously been infected with Chlamydia pneumoniae and has entered the recovery period.
Product Features
1.Easy handling, no instrument required.
2. Results at 15 minutes.
3. Results are clearly visible and reliable.
4. High Accuracy.
5. Room temperature storage.
Specification
Product Name: Chlamydia pneumoniae IgG/IgM Test Kit
Package Specification: 25/30 Tests/Box
Storage Condition: The test kit can be stored at room temperature or refrigerated (2-30℃) for the duration of the shelf life.
Test Procedure
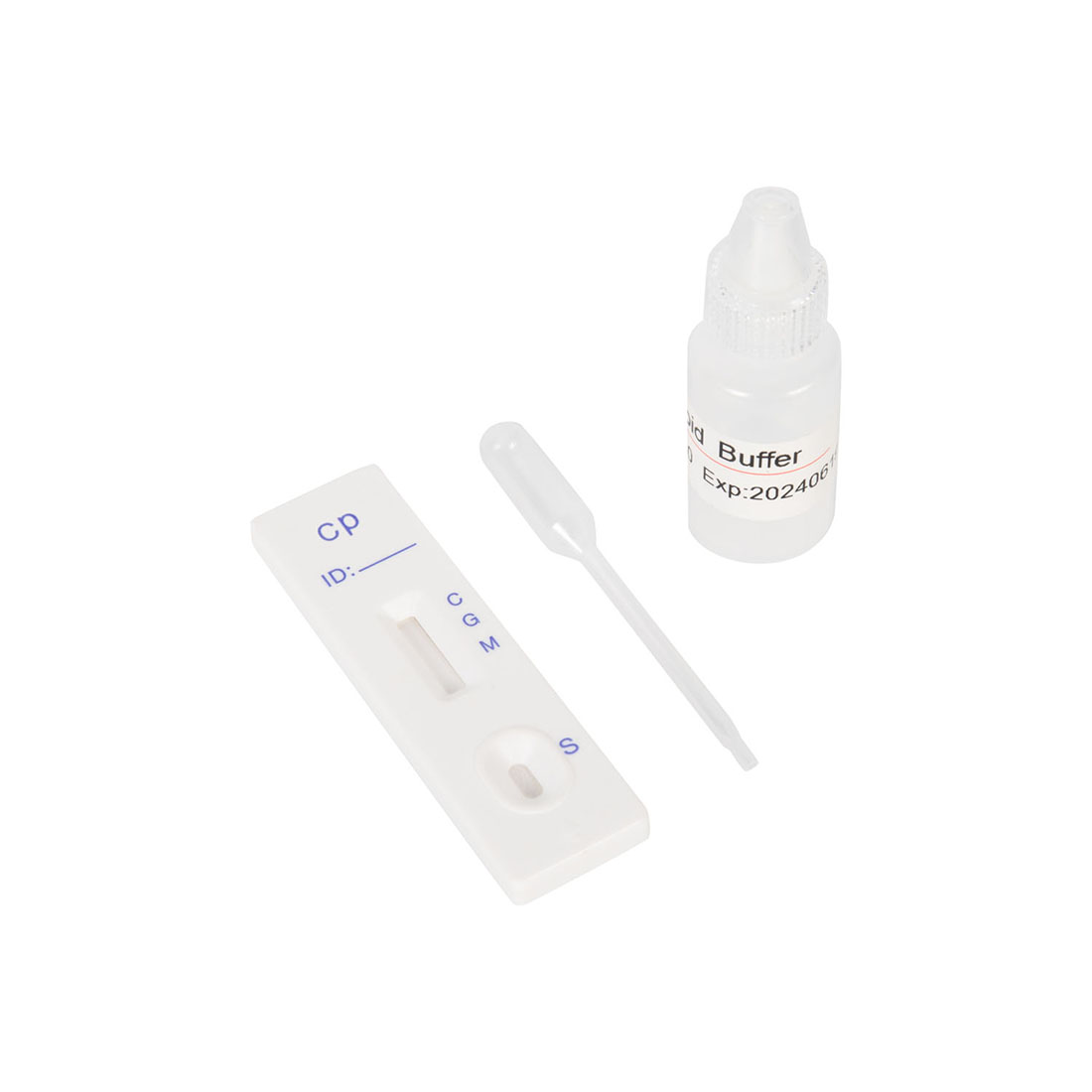
1.Bring all materials, including specimen and test device, recover to 15-25ºC before running the assay.
2.Remove the test device from the foil pouch and place it horizontally.
3.Using the disposable dropper, place 1 drop of sample into sample hole "S" of the test device. Immediately place 2 drops of assay buffer into the sample hole.
4.Interpret the result in 10-15 minutes. Result after 15 minutes is considered as invalid.
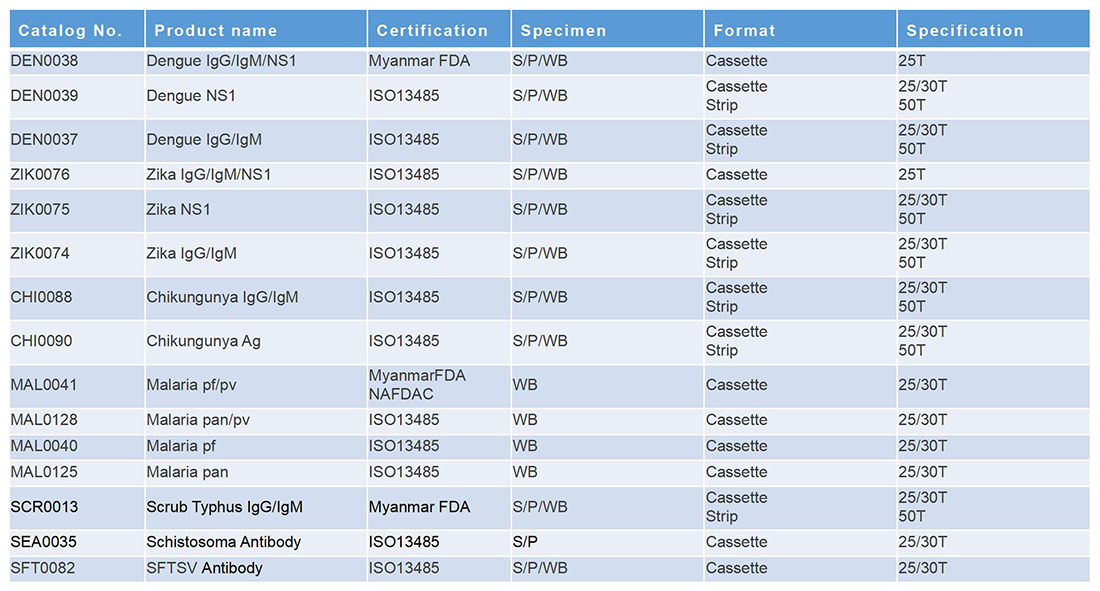
常见传染病
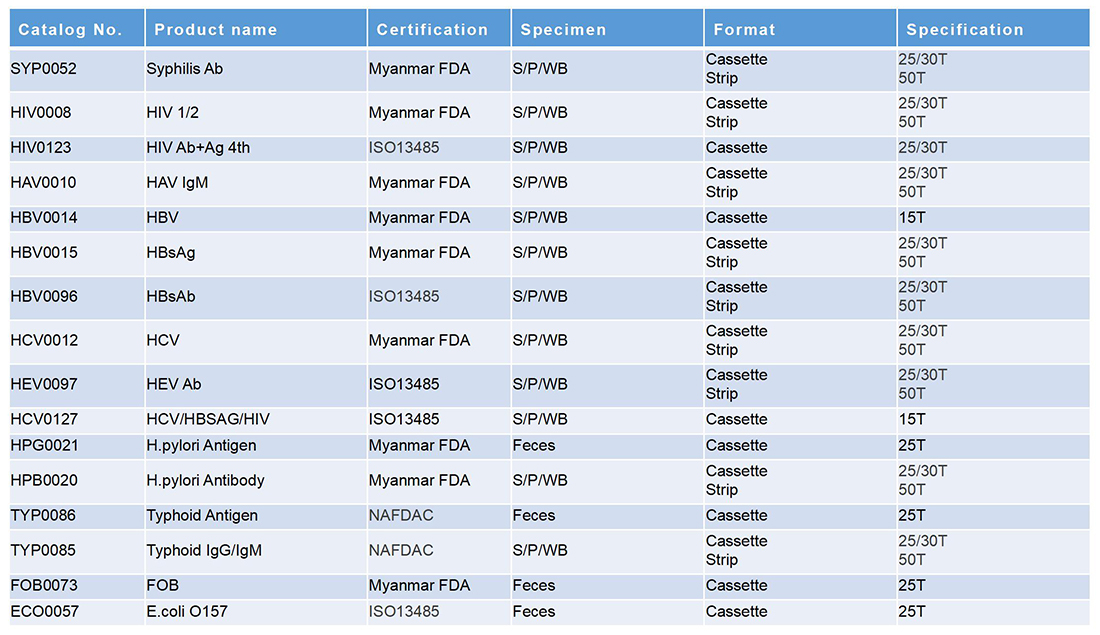
传染病
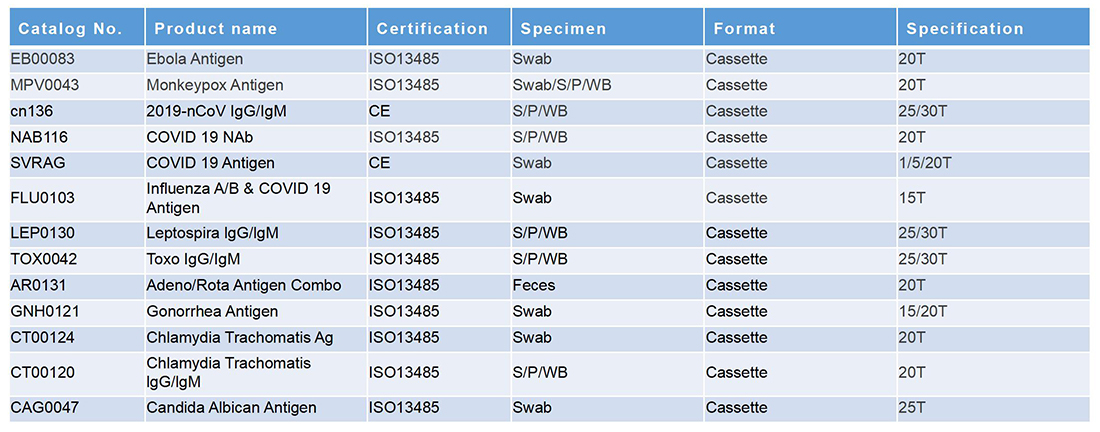
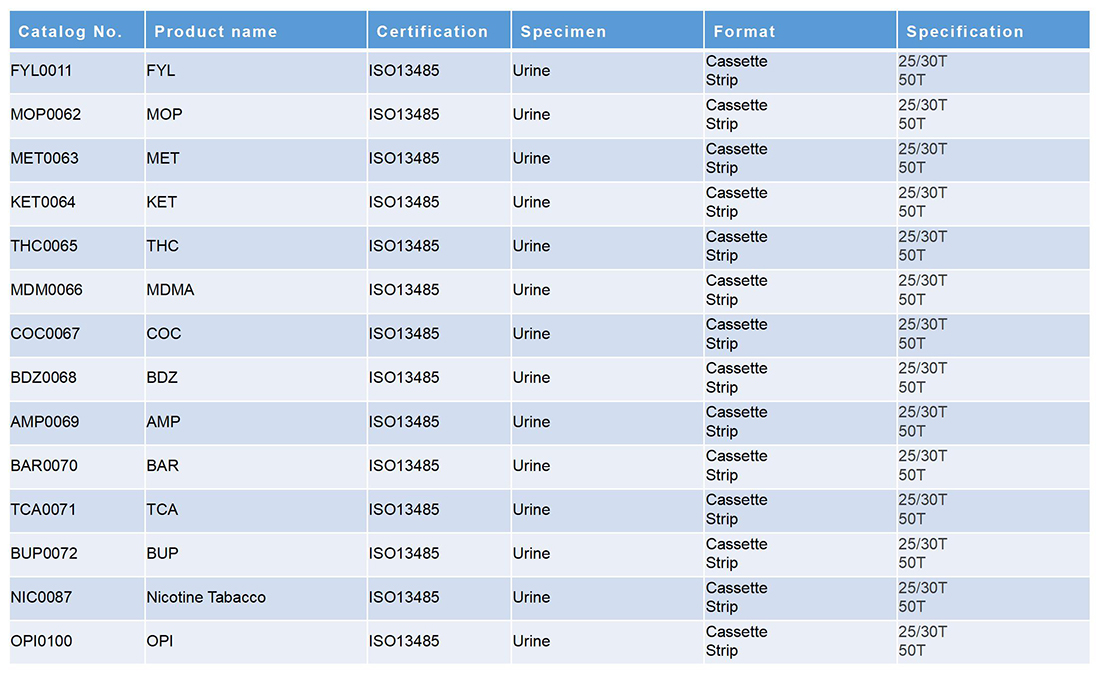
毒品列表
热带传染病
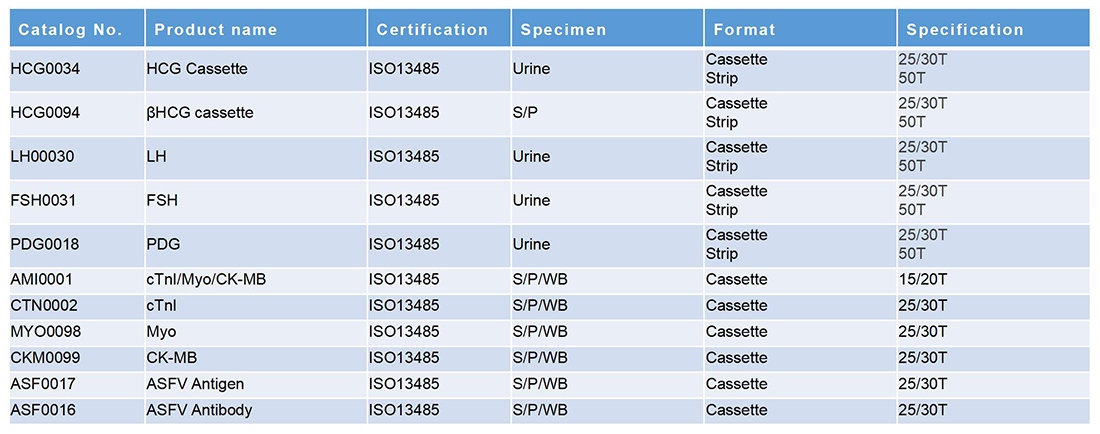
早孕+心肌
下一篇:没有了

