Welcome to the official website of Sinos (Suzhou) Bioengineering Co., Ltd!
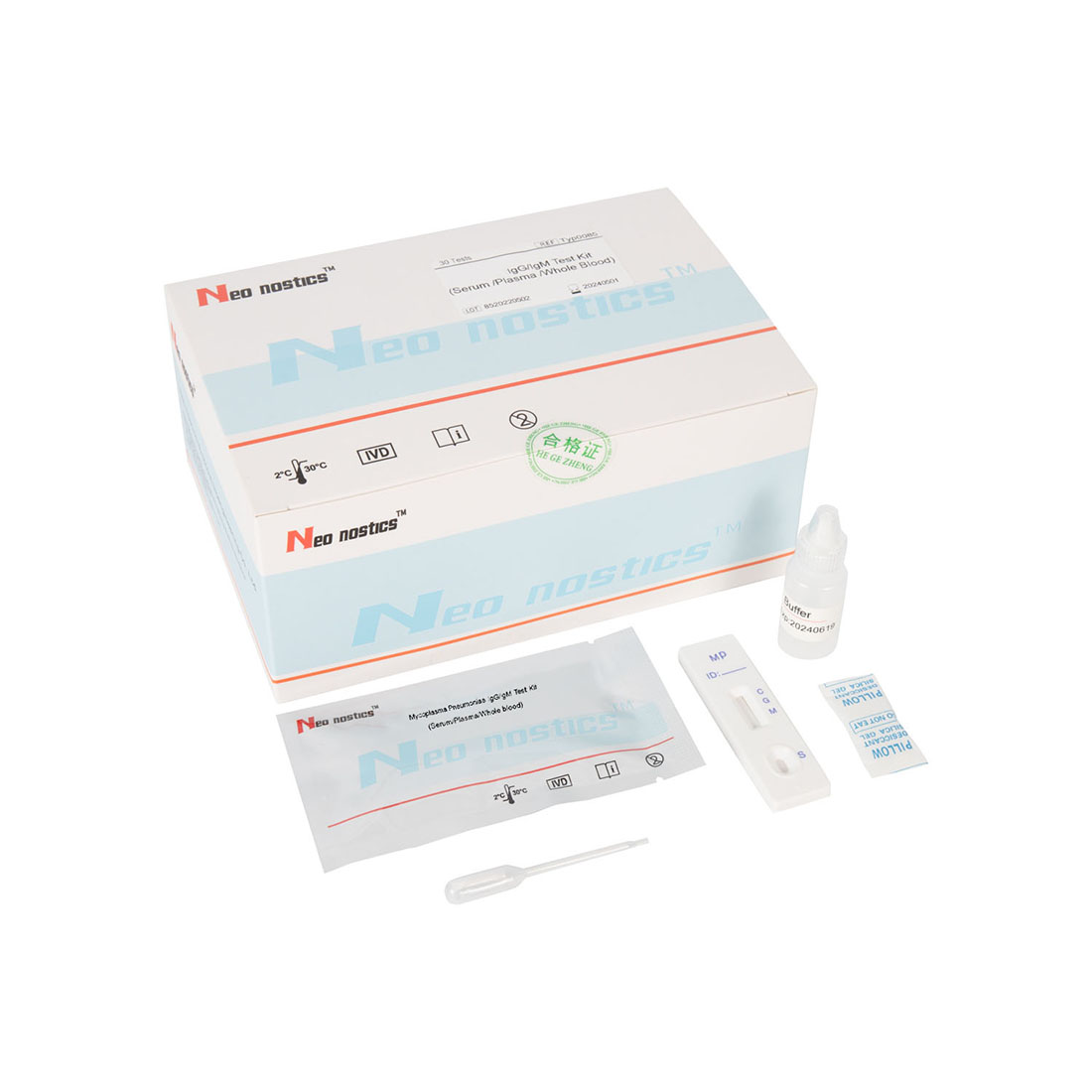
Mycoplasma pneumoniae IgG IgM(Colloidal Gold Method)
date:2023-12-19 23:35
Introduction
Mycoplasma pneumoniae (MP) is a common pathogen causing acute respiratory infections in adolescents.
After infection with Mycoplasma pneumoniae, the human body produces MP IgM, MP lgA, and MP IgG antibodies. MP IgM antibodies appear 4-5 days after the first infection and can be used as a diagnostic indicator for recent infections of Mycoplasma pneumoniae. The MP-lgA antibody rapidly increases in the early stage of Mycoplasma pneumoniae infection, produced by the conversion of MP-lgM type, and its changes are basically consistent with MP-lgM. The appearance time of MP IgG antibodies is delayed compared to MP lgM and MP IgA, usually appearing around 14 days after infection, reaching a peak value at 5 weeks and maintaining it for a long time. It can be used as a diagnostic indicator for previous infections of Mycoplasma pneumoniae. However, the clinical significance of detecting MP lgG alone is not significant, and it can be used for epidemiological investigations of previous infections of Mycoplasma pneumoniae.
In summary, the detection of Mycoplasma pneumoniae antibodies belongs to the detection of Mycoplasma pneumoniae antibodies. Qualitative detection of Mycoplasma pneumoniae antibodies or quantitative detection of antibody titers can be used to diagnose whether Mycoplasma pneumoniae is infected. Non infected individuals with Mycoplasma pneumoniae cannot detect MP lgM antibodies, MP IgA, and MP IgG antibodies in their serum.
Product Features
1.Easy handling, no instrument required.
2. Results at 15 minutes.
3. Results are clearly visible and reliable.
4. High Accuracy.
5. Room temperature storage.
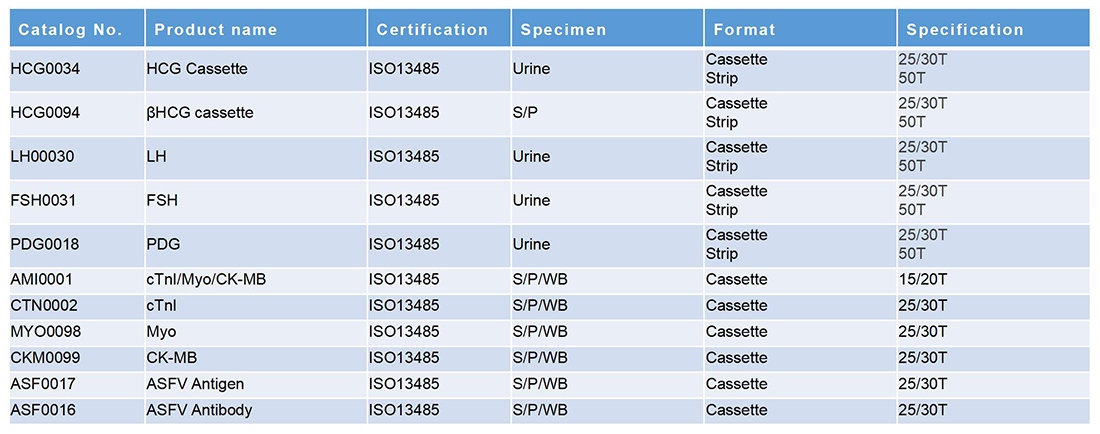
Specification
Product Name: Mycoplasma pneumoniae IgG/IgM Test Kit
Package Specification: 25/30 Tests/Box
Storage Condition: The test kit can be stored at room temperature or refrigerated (2-30℃) for the duration of the shelf life.
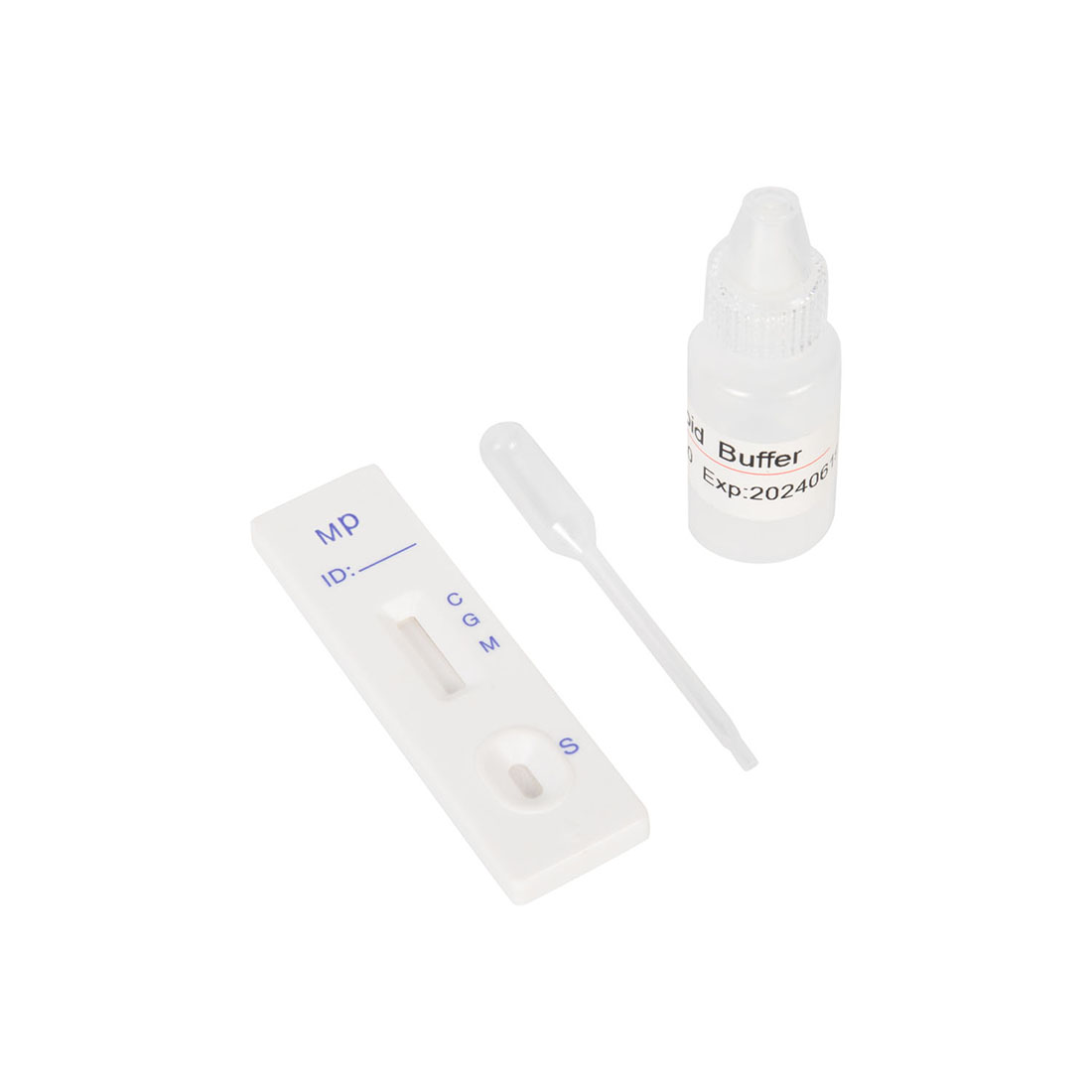
Test Procedure
1.Bring all materials, including specimen and test device, recover to 15-25ºC before running the assay.
2.Remove the test device from the foil pouch and place it horizontally.
3.Using the disposable dropper, place 1 drop of sample into sample hole "S" of the test device. Immediately place 2 drops of assay buffer into the sample hole.
4.Interpret the result in 10-15 minutes. Result after 15 minutes is considered as invalid.
常见传染病
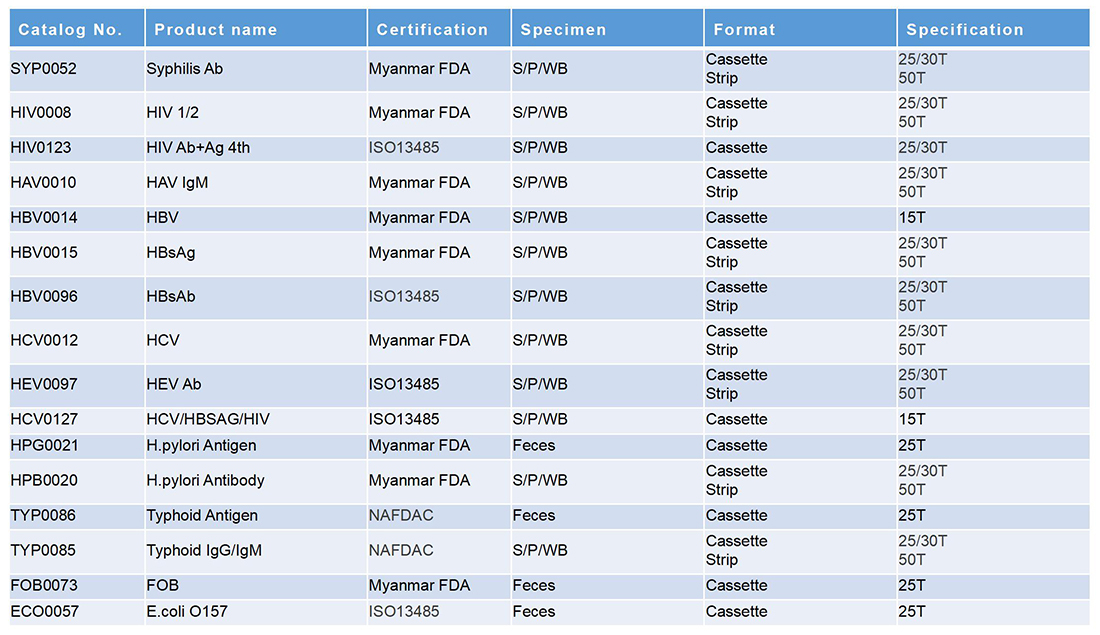
传染病
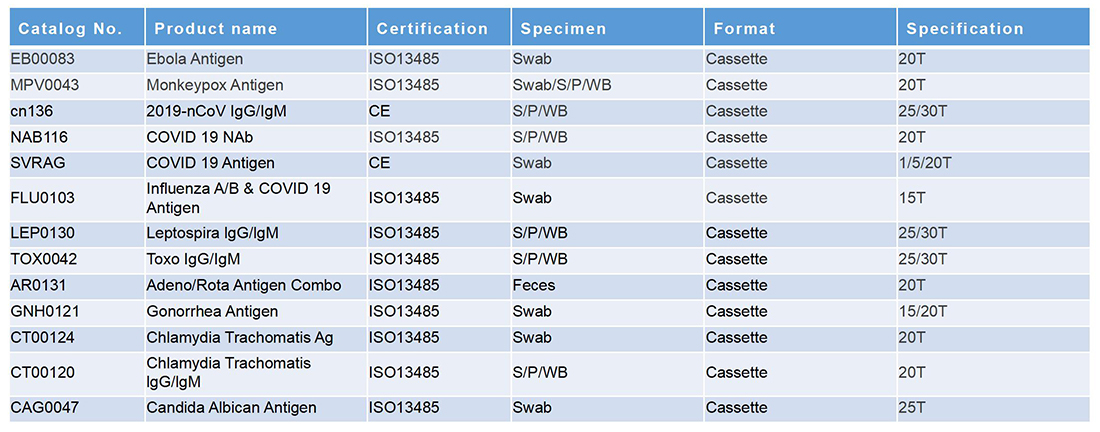
毒品列表
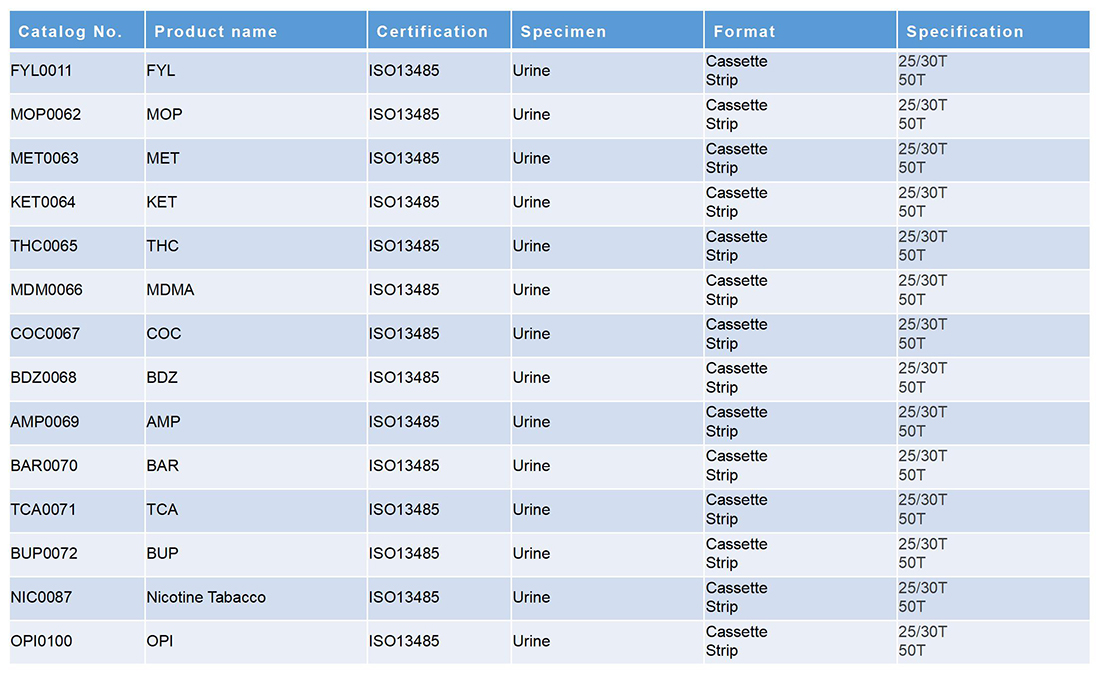
热带传染病
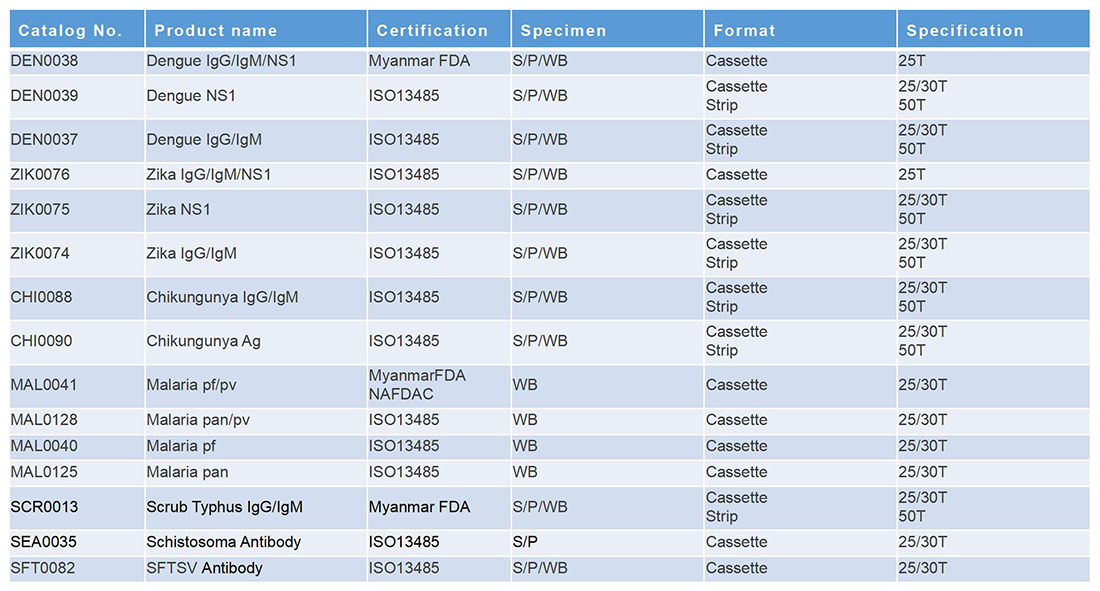
早孕+心肌